Thermablate EAS is a minimally invasive one time treatment option for pre menopausal women suffering from heavy menstrual bleeding due to begin causes who have completed childbearing

Patient Selection
Proper patient selection is very important to consider. Not all women can undergo a Thermablate treatment. Patients should always be evaluated thoroughly prior to treatment to rule out any underlying causes of their excessive uterine bleeding.

Potential Thermablate patients must meet certain criteria to be eligible for treatment:
- Excessive uterine bleeding with no underlying causes
- Completed childbearing
- Pre-menopausal normal uterine cavity with sounding between 8cm and 12cm, inclusive
- Normal endometrial biopsy and pap smear in accordance with Clinical Practice Guidelines
Contraindications
It is important to rule out contraindications prior to treating a patient with the Thermablate system. Most importantly, patients should understand that becoming pregnant after a Thermablate procedure is possible but it may be unsafe for both the mother and the foetus. Proper birth control counselling should be provided to patient’s pre and post procedure. Repeat endometrial ablations should not be offered or attempted with Thermablate EAS as the uterine cavity is likely distorted.
- User slowly inserts catheter until balloon tip touches the fundus of the uterine cavity
- Depth markings on catheter must match previously obtained sounding measurements
- Treatment cycle is activated with a simple finger trigger switch
- Thermablate system automatically inflates and deflates the balloon to ensure consistent delivery of energy and contact with the endometrium
Benefits of the Balloon and Long Term Results
Proven long-term results demonstrate high efficacy and patient satisfaction, as well as low re-intervention in the form of hysterectomy(3).
At Two Years
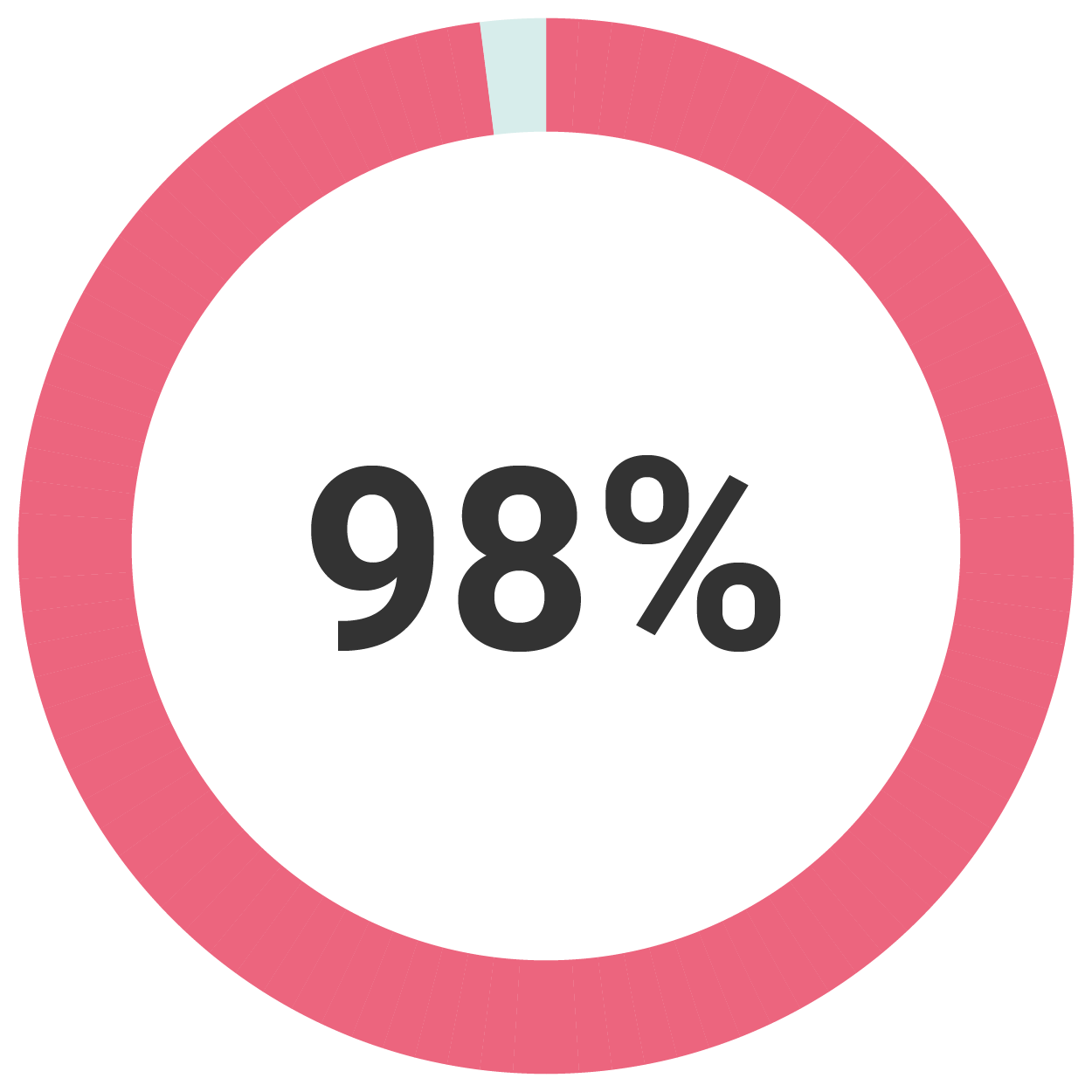
Up to 98% of patients reported return to normal menstrual bleeding, hypomenorrhea or amenorrhea(1)
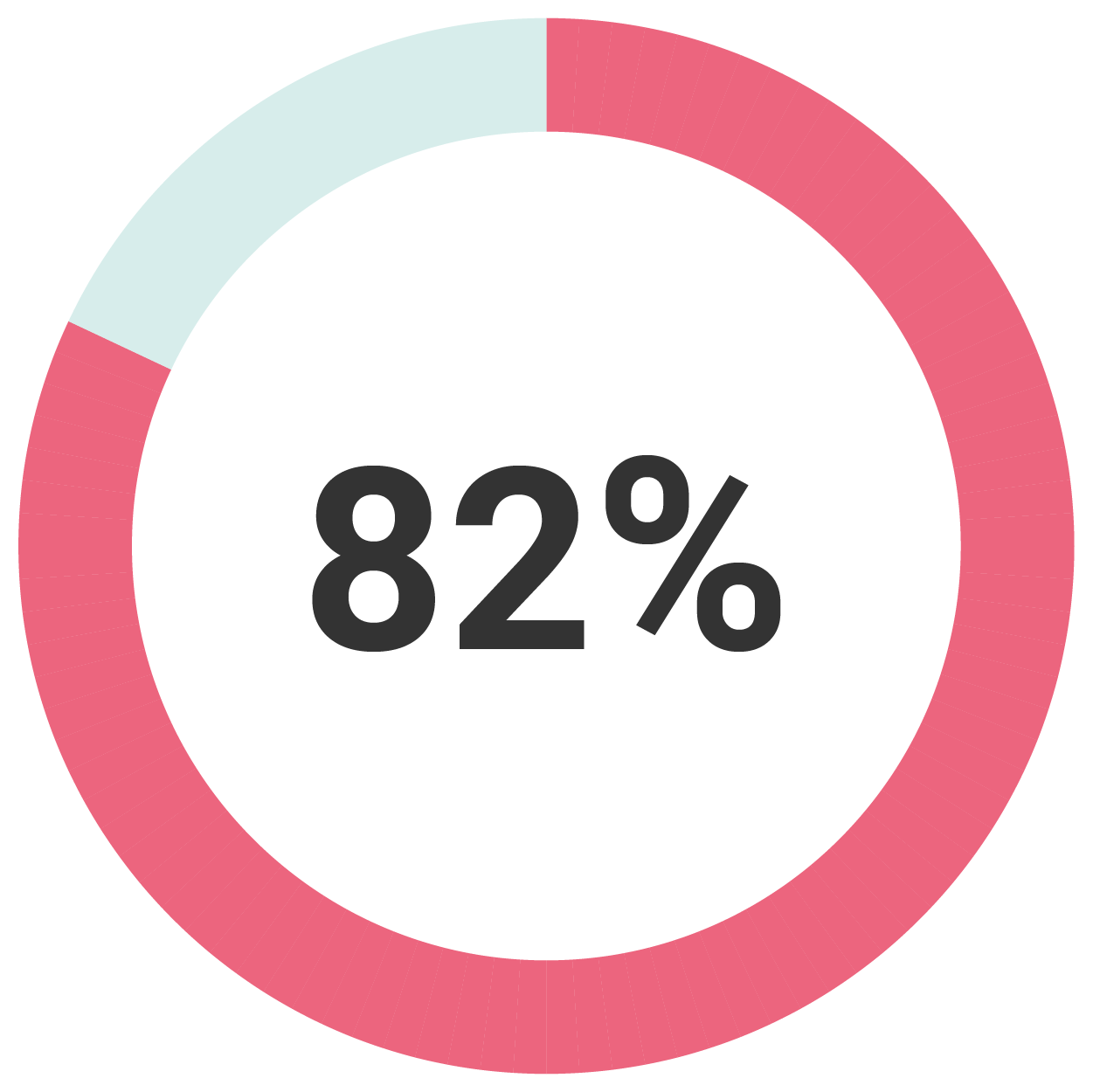
82% of women were satisfied with their treatment(2)
At a Median of Five Years
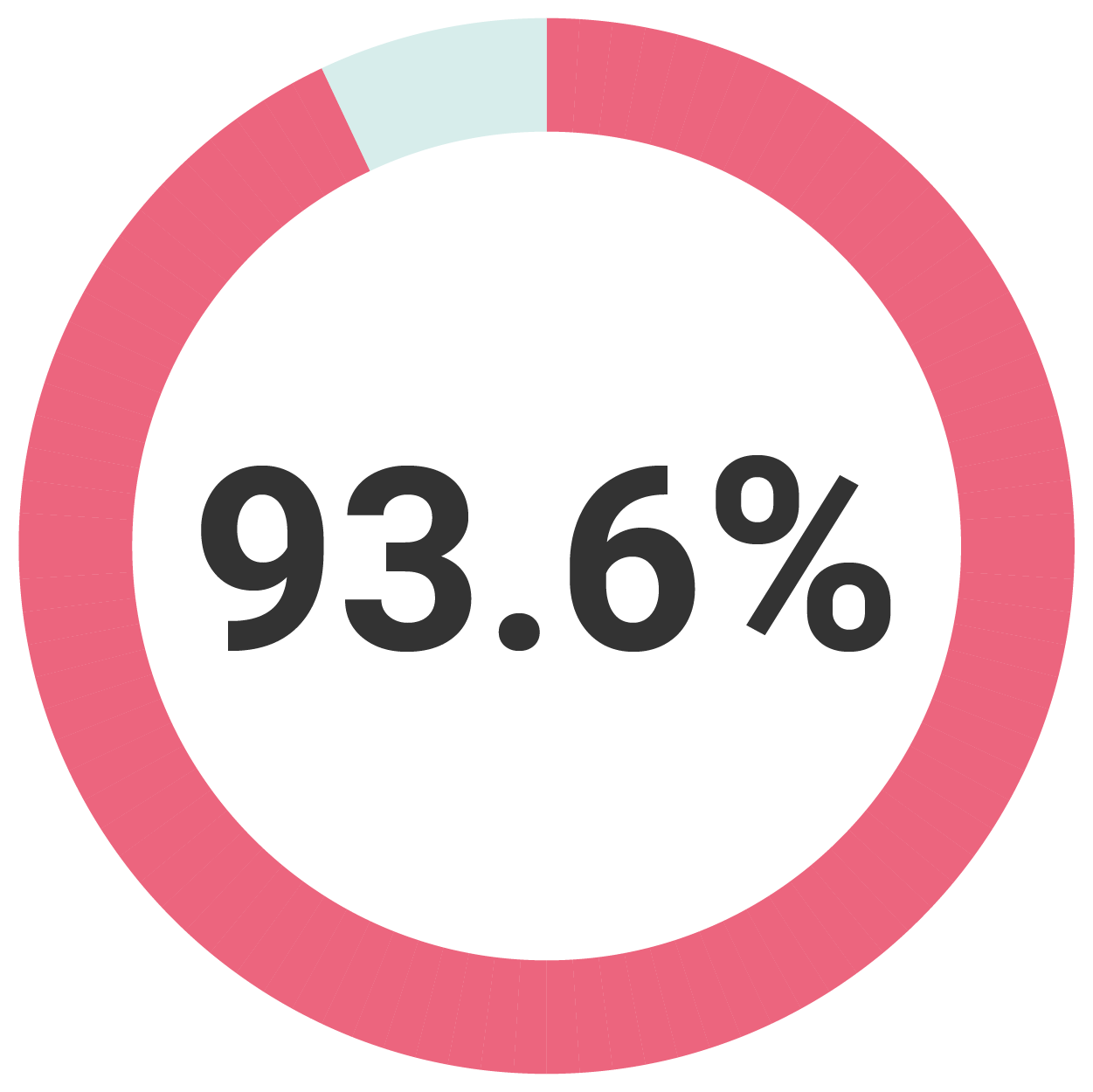
93.6% of women avoided hysterectomy(3)
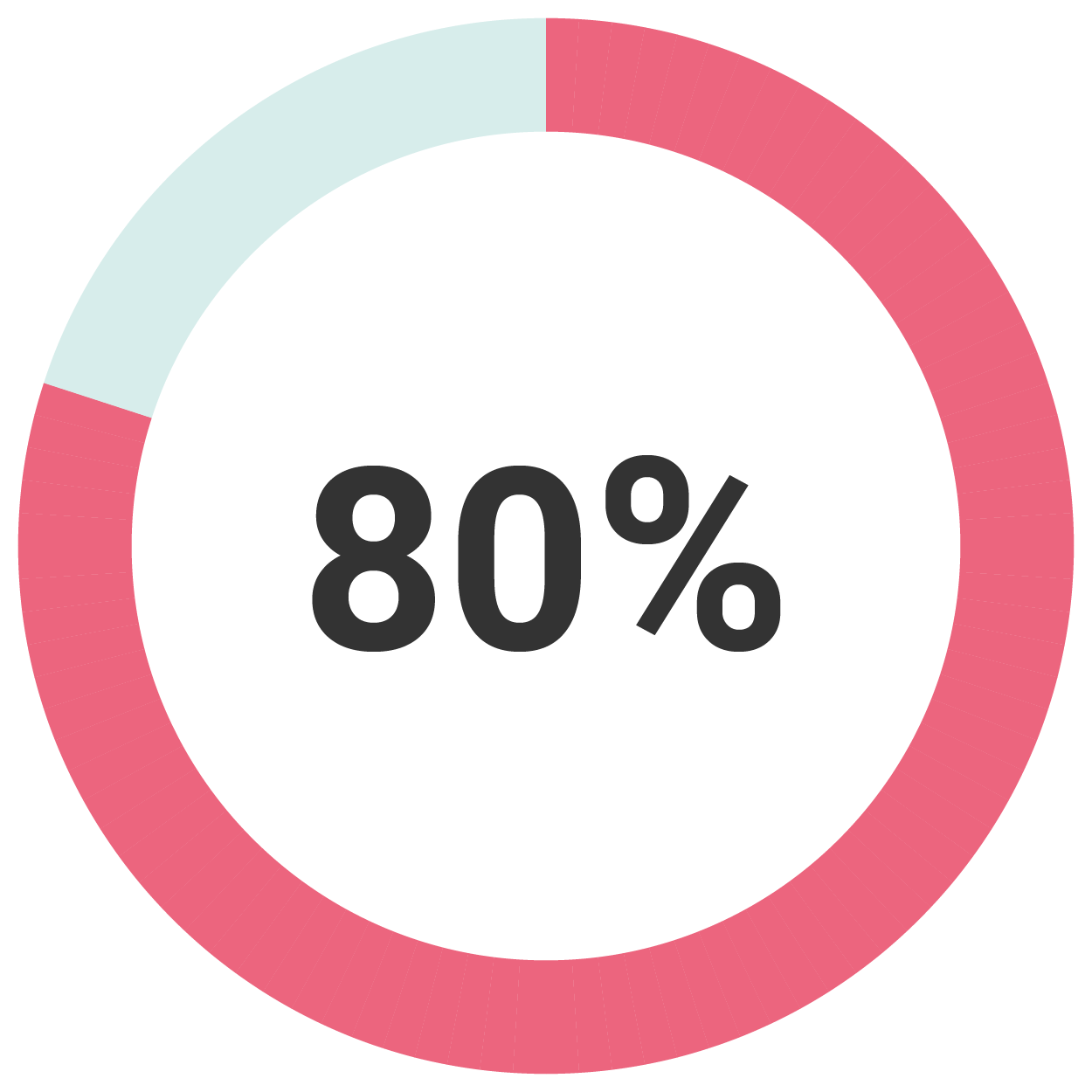
80% of patients experienced reduction in menstrual bleeding and required no additional therapy. 6.4% underwent hysterectomy(3)
Success Measured as Improved Quality of Life
Thermal balloon ablation offers many benefits over competitive Global Endometrial Ablation systems and is the ideal treatment option for avoiding long-term complications such as De Novo (newly onset) Pelvic Pain.
A study comparing the incidence of De Novo Pelvic Pain within 2 years of either Radiofrequency (RF) or Balloon Ablation found that:
- More focus is being placed on improved quality of life rather than solely menstrual patterns post ablation
- The possibility of De Novo Pelvic Pain post-ablation should be reviewed with the patient pre-procedure
- The incidence as well as its associated severity varies by mode of therapy (RF>TB)(9)
“De Novo pelvic pain occurred overall in 20% of radiofrequency patients and 7% of Thermal Balloon patients”(9)
References
- D’Afiero A et al. Efficacy of a Second Generation Thermal Balloon Device in the Treatment of Anemia Induced by Meno/Metrorrhagia. International Journal of Gynecology & Obstetrics 2012; S261-S530.
- Karamanidis D et al. Two Year Results of a New Two Minute Hot Liquid Balloon Endometrial Ablation System (Thermablate): A Pilot Study. Clinical and Experimental Obstetrics & Gynecology 2009; 36(4): 256-258.
- Qaiser A, Chen BF, Powell MC. A Long Term Follow up of Results of Women undergoing an Office Based Thermablate Endometrial Ablation for the Treatment of Menorrhagia. Obstet Gynecol Int J 2016, 4(5): 00127.
- Leyland N. Office Based Global Endometrial Ablation: Feasibility and Outcome for 3 Modalities. Journal of Obstetrics and Gynaecology Canada 2004; 26:S22.
- Hall M, Woodward Z. Outpatient Endometrial Ablation: Patient Reported Efficacy and Acceptability. Royal College of Obstetricians and Gynaecologists World Congress 2016; Poster Presentation.
- Prasad P, Powell M. Prospective Observational Study of Thermablate Endometrial Ablation System as an Outpatient Procedure. J Min Invas Gynecol 2008; 15:476-479.
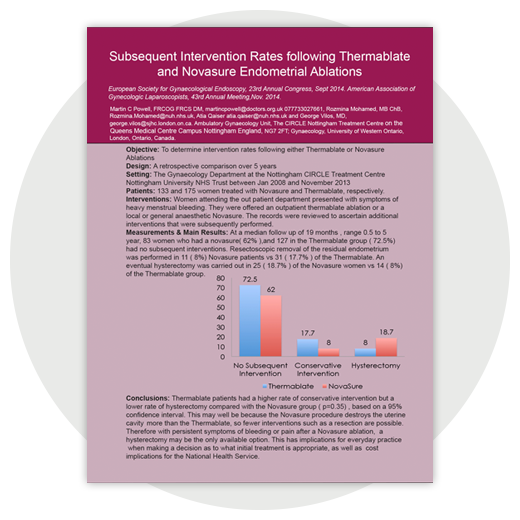
- Powell M et al. Subsequent Intervention Rates Following Thermablate and Novasure Endometrial Ablations. J Min Invas Gynecol 2014; 21:S136-S190.
- Longinotti MK, Jacobson GF, Hung Y, Learman LA. Probability of Hysterectomy After Endometrial Ablation. Obstet Gynecol 2008; 112:1214-1220.
- Chapa H, Antonetti A, Sandate J, Bakker K, Silver L. Incidence of de Novo Pelvic Pain After Radiofrequency or Thermal Balloon Global Endometrial Ablation Therapy. J Gynecol Surg 2011; 27(4): 203-207.
DOWNLOAD CLINICAL PAPERS
The Concomitant Use of the Two Minute Thermal Balloon Endometrial Ablation (Thermablate EASTM) System and the Levonorgestrel Intra-uterine System (LNG-IUS) Significantly Improves Clinical Outcomes in Women with Heavy Menstrual Bleeding
Vilos, Vilos, Marks, Oraif et al / Society of Obstetrics & Gynaecologists of Canada, 70th Annual Meeting, June 2014
To learn more about Thermablate EAS, please visit our manufacturer partner website here
CONTACT US TODAY